News
Semaglutide's new indication for chronic kidney disease applied for listing in China
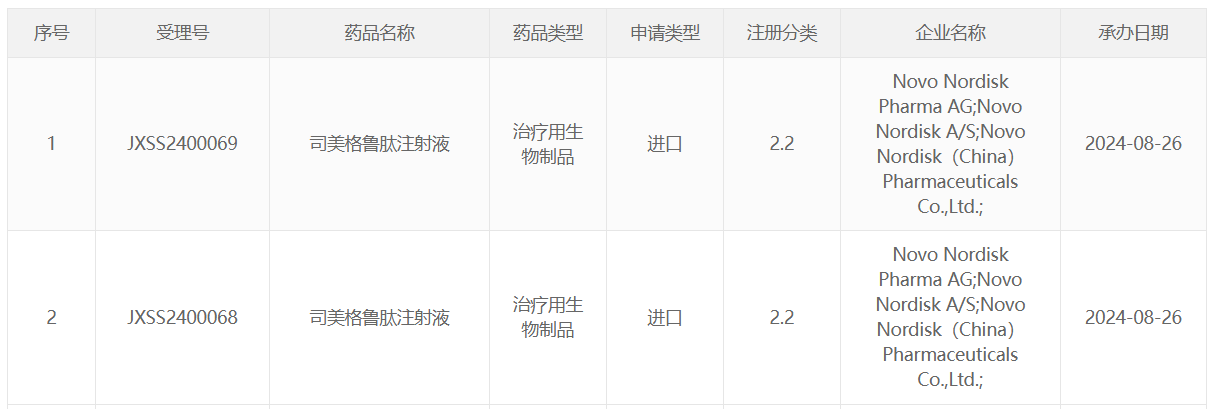
On August 26, 2024, the official website of the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) announced that Novo Nordisk's application for the new indication of semaglutide injection has been officially accepted by the NMPA, and it is speculated that this application is for indications related to chronic kidney disease (CKD).
semaglutide is a long-acting GLP-1 analogue that shares 94% homology with the amino acid sequence of natural GLP-1 and acts as a GLP-1 receptor agonist. Smeaglutide 0.25, 0.5 and 1.0 mg injections were approved in China in April 2021 for the treatment of type 2 diabetes (trade name: Novotel, Ozempic); Smeaglutide 2.4 mg injections (trade name: Novotel, Wegovy) were approved in China in June 2024 for long-term weight management.
According to the query on the official website of the Chinese drug clinical trial and information publicity platform, Novo Nordisk has also carried out a number of phase 3 clinical studies on semaglutide injection, including an international multi center (including China) phase 3 clinical study (FLOW study) to evaluate the role of semaglutide in the progression of renal damage in patients with type 2 diabetes combined with chronic kidney disease; An international multicenter (including China) phase 3 clinical study was conducted to evaluate the effect of semaglutide on the functional ability of patients with type 2 diabetes and peripheral arterial disease; An international multicenter (including China) Phase 3 clinical study evaluating the effect of semaglutide on subjects with non cirrhotic non-alcoholic fatty liver disease.
In October 2023, Novo Nordisk announced that the Phase III clinical FLOW of semaglutide in the treatment of patients with type 2 diabetes and chronic kidney disease with renal insufficiency would be terminated early due to its excellent efficacy.
The FLOW study is a randomized, double-blind, placebo-controlled, superiority test (n=3533) to evaluate the efficacy and safety of semaglutide (1.0mg) and placebo as adjunctive treatment schemes for standard treatment of kidney outcomes to prevent the progression of kidney injury in type 2 diabetes patients with CKD and reduce the risk of kidney and cardiovascular death.
The results showed that compared with the placebo group, patients in the semaglutide group had a 24% reduction in renal disease progression and cardiovascular and renal mortality risk, achieving the efficacy endpoint. In addition, the study also met the criteria for superiority in terms of confirmatory secondary endpoints. In terms of safety, semaglutide has good safety and tolerability, consistent with previous studies.
CATEGORIES
News
- Welcome to the world of Tirzepatide, a w2025-02-28
- Is compound semaglutide safe?2025-02-25
- An average weight loss of over 20%! Tirz2025-02-24
- World Health Organization: GLP-1 such as2025-02-20
- Fortune: 'Weight loss miracle drug' sema2025-02-18
CONTACT US
Contact: NewPeptides
Phone: +852 6902 7583
E-mail: Linda@goodpeptides.com
Add: Science and Technology Industrial Park, Yuelu District, Changsha City, Hunan Province
