News
Qilu Pharmaceutical Co., Lplies for market latd. apunch of Semaglutide Injection
Editor of the Peptide Biochemistry Content Team
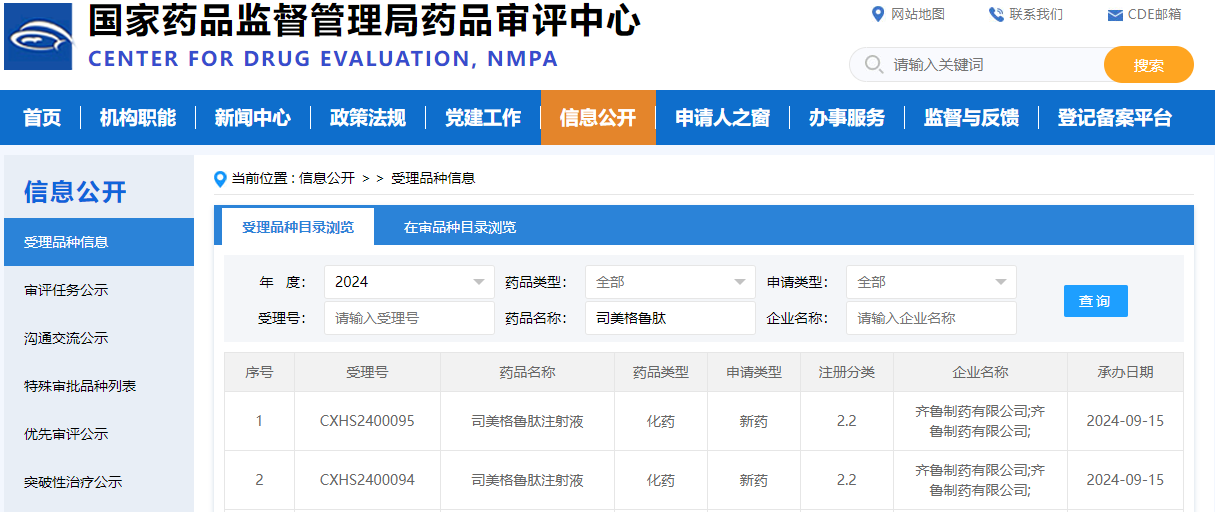
On September 15, 2024, the China National Medical Products Administration's Center for Drug Evaluation (CDE) platform showed that Qilu Pharmaceutical's application for the marketing of semaglutide injection had been accepted, but the specific indications have not yet been disclosed. According to the clinical study of Smeaglutide injection carried out by Qilu Pharmaceutical, the indication of this declaration may be type 2 diabetes.
In the first half of 2024, the sales of Ozempic, a hypoglycemic injection version of semaglutide, reached 8.287 billion US dollars, a year-on-year increase of 36%; The sales revenue of Rybelsus, a hypoglycemic oral version of semaglutide, reached 1.598 billion US dollars, a year-on-year increase of 32%; The sales of the semaglutide weight reducing version of Wegovy reached 3.075 billion US dollars, a year-on-year increase of 74%. The total sales revenue of semaglutide is 12.96 billion US dollars. Accounting for two-thirds of Novo Nordisk's total revenue.
About semaglutide
semaglutide is a GLP-1 receptor agonist that stimulates insulin production and inhibits glucagon secretion, reducing appetite and food intake. Smeaglutide was initially approved for marketing as a therapeutic drug for type 2 diabetes (trade name: Ozempic). In view of its remarkable effect in weight loss, FDA approved it for the treatment of ordinary obese patients (trade name: Wegovy) in June 2021. It is the first new drug approved by the US FDA to control ordinary obesity or overweight since 2014. The drug was later approved by the European Union for the treatment of obesity in the same year.
CATEGORIES
News
- Welcome to the world of Tirzepatide, a w2025-02-28
- Is compound semaglutide safe?2025-02-25
- An average weight loss of over 20%! Tirz2025-02-24
- World Health Organization: GLP-1 such as2025-02-20
- Fortune: 'Weight loss miracle drug' sema2025-02-18
CONTACT US
Contact: NewPeptides
Phone: +852 6902 7583
E-mail: Linda@goodpeptides.com
Add: Science and Technology Industrial Park, Yuelu District, Changsha City, Hunan Province
