News
The new indication of Tirzepatide Dipeptides has been applied for listing in China
On September 15, 2024, the China National Medical Products Administration's Center for Drug Evaluation (CDE) platform announced that Eli Lilly and Company's application for a new indication for the GIP/GLP-1 receptor dual agonist, Tirzepatide injection, has been accepted, but the specific indications have not yet been disclosed. According to the CDE official website, this is the third listing application submitted by Tirzepatide in China.
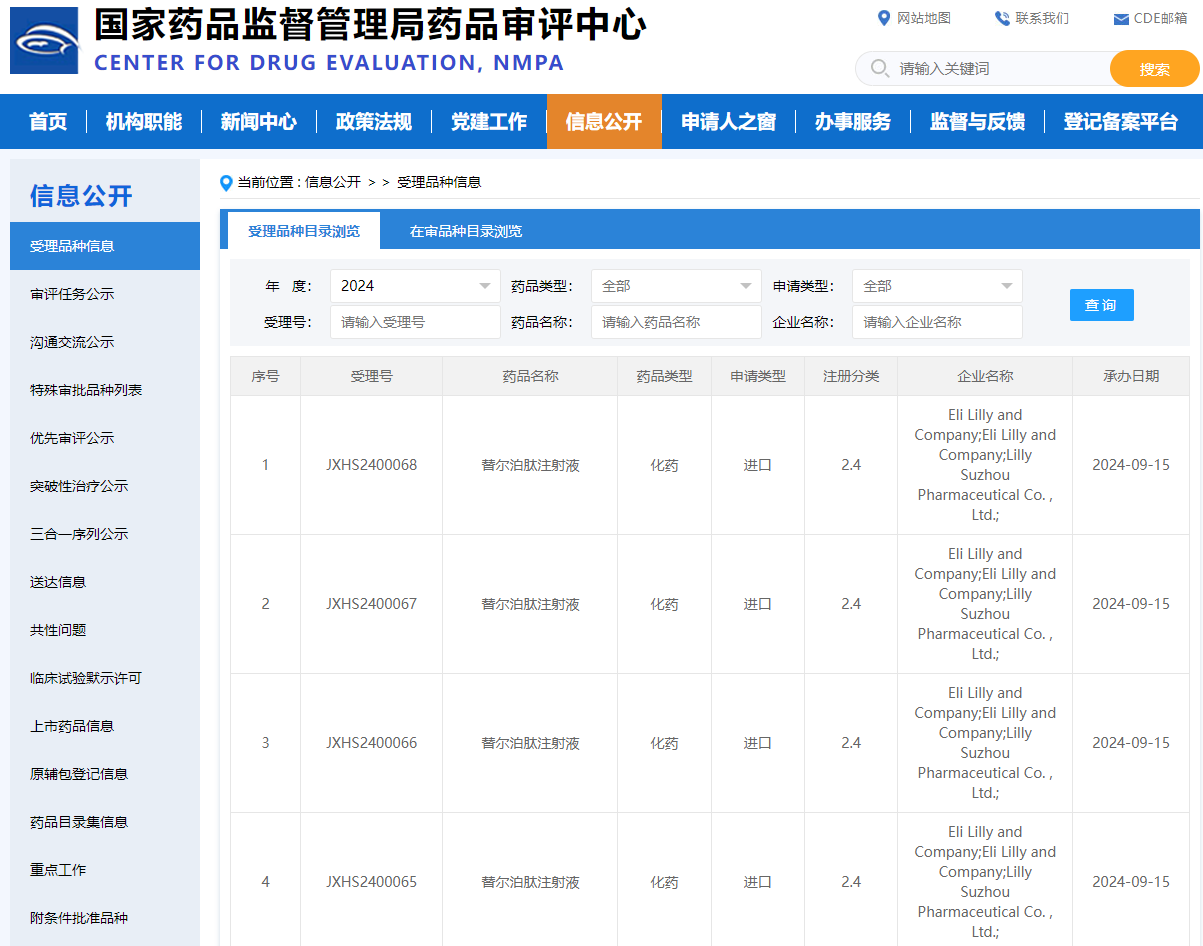
Based on the international registration progress of the product and the clinical research progress in China, it is speculated that the indication for this application for market launch may be to improve snoring in obese patients with moderate to severe obstructive sleep apnea (OSA). Regarding this indication, Lilly has submitted a marketing application to the FDA. In addition, the international multicenter (including China) Phase 3 clinical trial of Tirzepatide for the indication of preserved ejection fraction heart failure in patients with obesity has been completed and has achieved positive results.
About Tirzepatide
Tirzepatide is a dual agonist of glucose dependent insulinotropic polypeptide (GIP) and glucagon like peptide-1 (GLP-1) receptor, which can simultaneously activate GLP-1 receptor and GIP receptor-mediated signaling pathways. GIP and GLP-1 are natural intestinal insulinotropic hormones that regulate blood sugar. Tirzepatide was approved by the US FDA (trade name: Mounjaro) in May 2022 for use in combination with diet control and exercise to improve the blood sugar control of adult type 2 diabetes patients. Last November, tirzepatide received FDA approval again (trade name: Zepbound) for the purpose of reducing weight and maintaining weight stability in obese or overweight adult patients.
Eli Lilly and Company is a globally leading pharmaceutical company engaged in drug research and development, production, and sales, committed to improving human health through innovation. Eli Lilly was founded over a century ago by Colonel Eli Lilly in Indiana, USA in 1876. The company's founder was dedicated to producing high-quality drugs to meet practical medical needs.
CATEGORIES
News
- Welcome to the world of Tirzepatide, a w2025-02-28
- Is compound semaglutide safe?2025-02-25
- An average weight loss of over 20%! Tirz2025-02-24
- World Health Organization: GLP-1 such as2025-02-20
- Fortune: 'Weight loss miracle drug' sema2025-02-18
CONTACT US
Contact: NewPeptides
Phone: +852 6902 7583
E-mail: Linda@goodpeptides.com
Add: Science and Technology Industrial Park, Yuelu District, Changsha City, Hunan Province
