News
Semaglutide's indication for heart failure has been recommeennded and approved by European regulatory agcies
On September 20, 2024, Novo Nordisk announced that 2.4mg Wegovy (semaglutide) has been recommended and approved by the European Medicines Agency's Committee for Human Use (CHMP) for the treatment of obesity related ejection fraction preserved heart failure (HFpEF). This drug is the first GLP-1 drug recommended and approved for the treatment of heart failure.

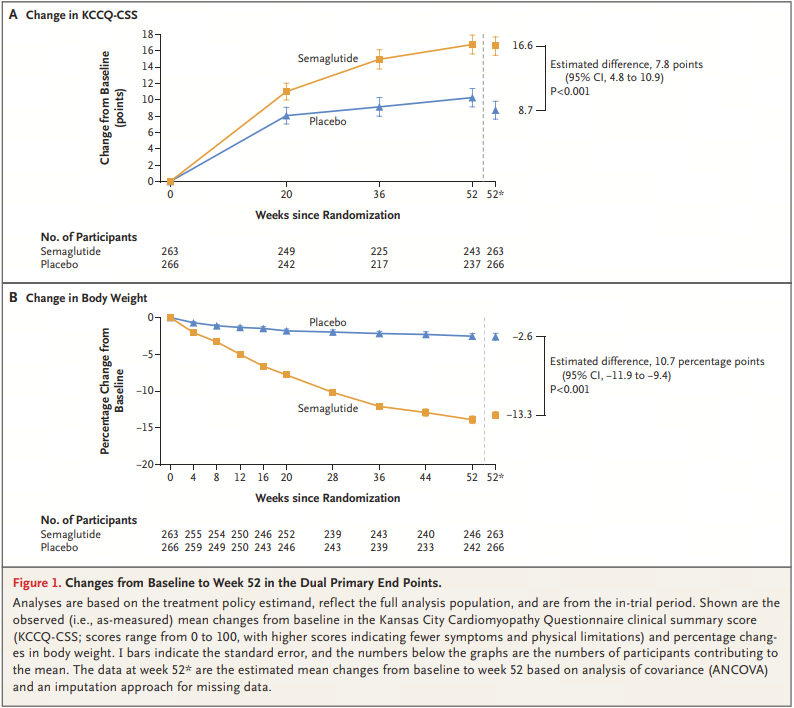
The CHMP's recommendation for approval this time is mainly based on the positive results of two Phase III studies (STEP HFpEF and STEP HFpEF DM). In the placebo-controlled STEP HFpEF study (n=529), after 52 weeks of treatment, 2.4mg Wegovy not only reduced the weight of obese patients with HFpEF without type 2 diabetes (-13.3% vs. -2.6%, p<0.001), but also reduced the symptoms related to heart failure (p=0.001). In addition, the patient's motor function has also significantly improved.
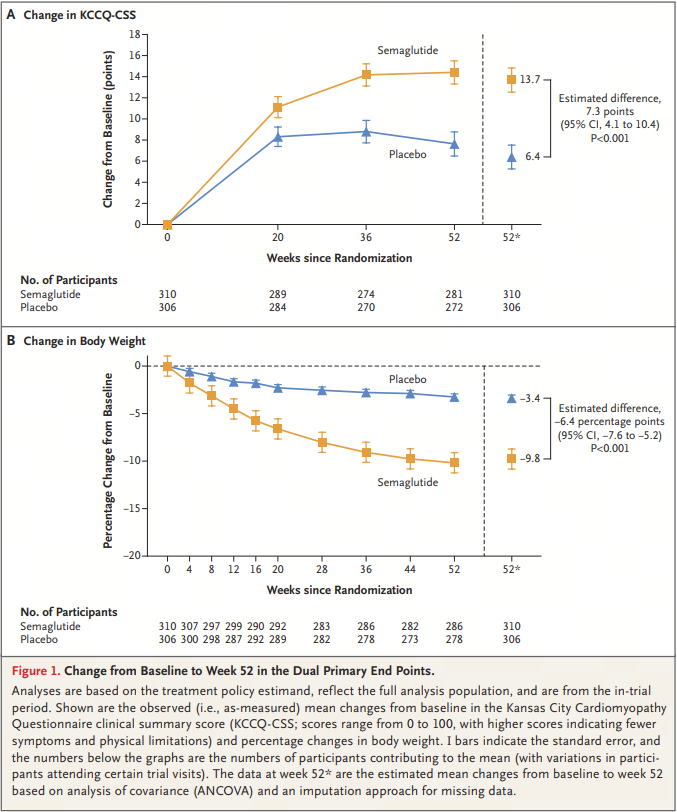
In the placebo-controlled STEP HFpEF-DM study (n=616), after 52 weeks of treatment, 2.4mg Wegovy not only reduced the weight of obese patients with HFpEF and type 2 diabetes (-9.8% vs. -3.4%, p<0.001), but also reduced the symptoms related to heart failure (p<0.001). In addition, the patient's motor function also significantly improved.
About Semaglutide
Semaglutide is a GLP-1 receptor agonist that stimulates insulin production and inhibits glucagon secretion, reducing appetite and food intake. Semaglutide was initially approved for marketing as a therapeutic drug for type 2 diabetes (trade name: Ozempic). In view of its remarkable effect in weight loss, FDA approved it for the treatment of ordinary obese patients (trade name: Wegovy) in June 2021. It is the first new drug approved by the US FDA to control ordinary obesity or overweight since 2014. The drug was later approved by the European Union for the treatment of obesity in the same year.
About Novo Nordisk
Novo Nordisk was founded in 1923 and is a globally leading biopharmaceutical company headquartered in Copenhagen, the capital of Denmark. Our goal is to promote change to combat diabetes, obesity, rare blood diseases, endocrine disorders and other serious chronic diseases. To achieve this goal, we lead scientific breakthroughs, expand the accessibility of our company's drugs, and are committed to preventing and ultimately curing diseases. Novo Nordisk has approximately 47000 employees in 80 countries and regions worldwide, providing products and services to over 168 countries and regions worldwide.
CATEGORIES
News
- Welcome to the world of Tirzepatide, a w2025-02-28
- Is compound semaglutide safe?2025-02-25
- An average weight loss of over 20%! Tirz2025-02-24
- World Health Organization: GLP-1 such as2025-02-20
- Fortune: 'Weight loss miracle drug' sema2025-02-18
CONTACT US
Contact: NewPeptides
Phone: +852 6902 7583
E-mail: Linda@goodpeptides.com
Add: Science and Technology Industrial Park, Yuelu District, Changsha City, Hunan Province
