News
Another potential new indication! semaglutide reappears in the New England Journal of Medicine
Knee osteoarthritis (OA) is one of the most common types of osteoarthritis, which can lead to chronic pain, reduced mobility, disability, and other issues. Obesity is an important factor in the occurrence and development of knee osteoarthritis. Previous studies have shown that for every 1% weight loss, pain, physical function, and hardness scores can be improved by 2% [based on the Western Ontario and McMaster University Knee Osteoarthritis Index (WOMAC)], and the risk of structural joint injury progression can be reduced. The current guidelines also recommend weight loss and physical activity as first-line treatment strategies for obesity related knee osteoarthritis. semaglutide is a common glucagon like peptide-1 (GLP-1) receptor agonist that has been approved for the treatment of obesity in multiple countries worldwide, but its role in obesity related knee osteoarthritis patients is not yet clear.
Recently, the New England Journal of Medicine (NEJM) published the research results of STEP9, which showed that in patients with obesity related knee osteoarthritis accompanied by moderate to severe pain, treatment with semaglutide once a week for 15.6 weeks can reduce weight by 10.5% compared to placebo, and WOMAC pain score can be reduced by 14.1 points (score range 0-50, the higher the score, the more severe the pain).

The STEP9 trial is a multicenter, double-blind, randomized, placebo-controlled trial conducted in 61 medical centers in 11 countries worldwide. The study included 407 adult patients with a body mass index (BMI) ≥ 30 kg/m2, diagnosed with knee osteoarthritis and moderate to severe pain. Researchers randomly assigned participants in a 2:1 ratio to receive subcutaneous injections of semaglutide once a week (271 cases) or placebo (136 cases). After 68 weeks of continuous treatment, an additional 7-week follow-up was conducted. The initial dose of semaglutide was 0.24 mg, gradually increasing to 2.4 mg at week 16. Throughout the entire trial, both groups of patients received low calorie diet and physical activity counseling.
At week 68 of treatment, semaglutide can significantly reduce body weight:
The body weight of the semaglutide group and the placebo group decreased by 13.7% and 3.2% respectively from baseline, with the semaglutide group showing a 10.5% increase in body weight compared to the placebo group (P<0.001).
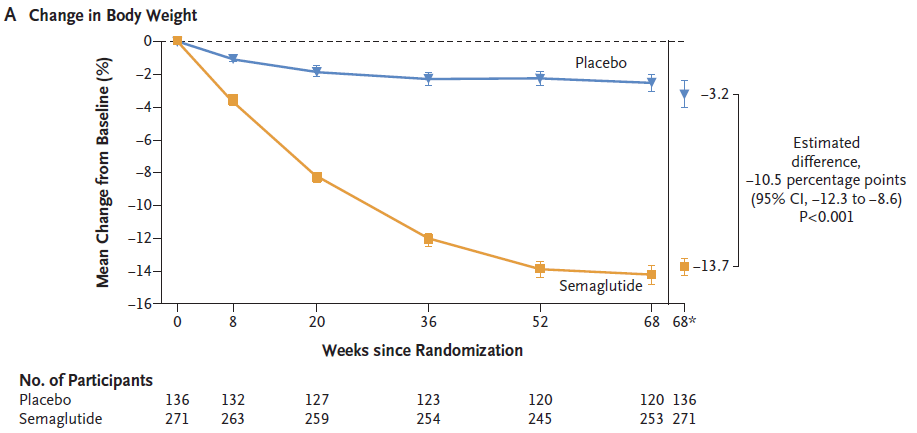
▲ Weight changes during treatment in the semaglutide group (orange) and placebo group (blue) (Image source: Reference [1])
The proportion of patients in the semaglutide group who had a weight loss of at least 5%, 10%, 15%, and 20% from baseline was 85.2%, 68.1%, 45.6%, and 22.3%, respectively, significantly higher than the placebo group's 33.6%, 12.9%, 4.5%, and 1.3% (all P<0.001).
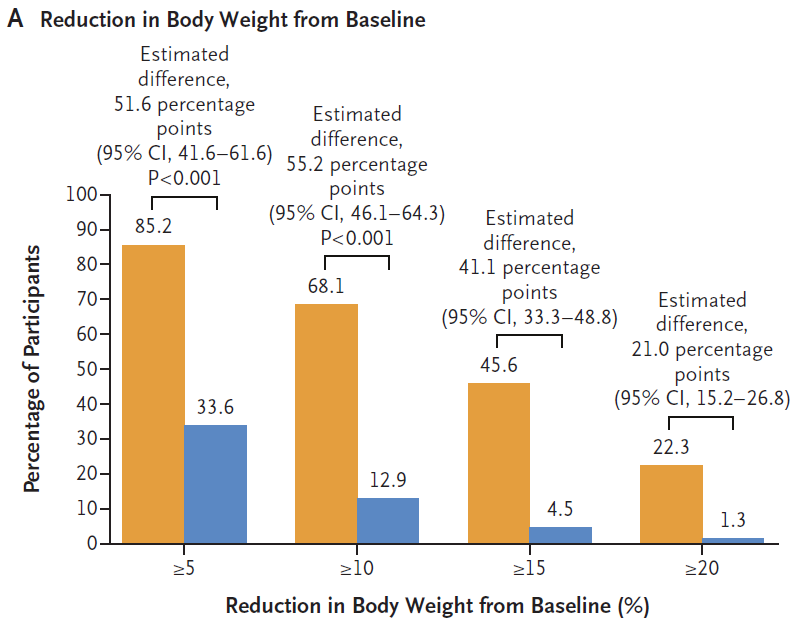
During the treatment period, the proportion of patients with weight loss greater than 5%, 10%, 15%, and 20% in the semaglutide group (orange) and placebo group (blue) (from left to right) (Image source: Reference [1])
At week 68 of treatment, semaglutide significantly improved WOMAC sub scores:
The pain scores of WOMAC in the semaglutide group and placebo group decreased by 41.7 points and 27.5 points, respectively, compared to baseline. The pain score of WOMAC in the semaglutide group decreased by 14.1 points more than that in the placebo group (P<0.001);
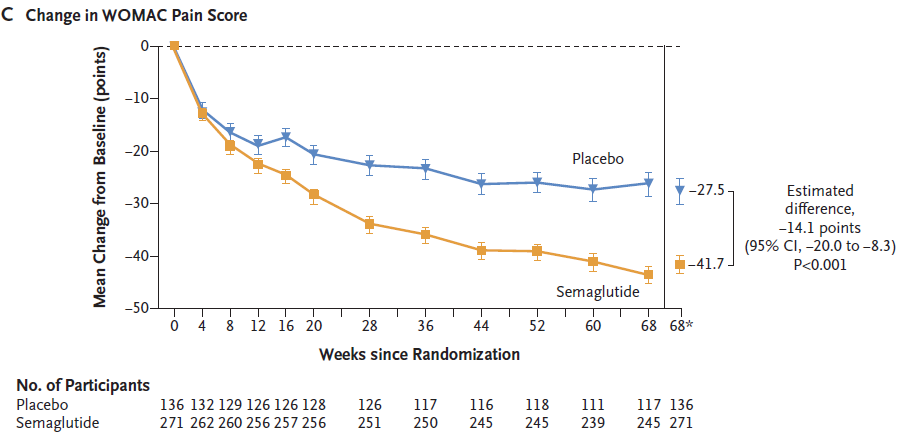
▲ Changes in WOMAC pain scores during treatment in the semaglutide group (orange) and placebo group (blue) (Image source: Reference [1])
The proportion of patients with a pain score reduction of at least 30% and 50% from baseline in the WOMAC group treated with semaglutide was 77.6% and 65.2%, respectively, which was significantly higher than the 57.8% and 35.3% in the placebo group.
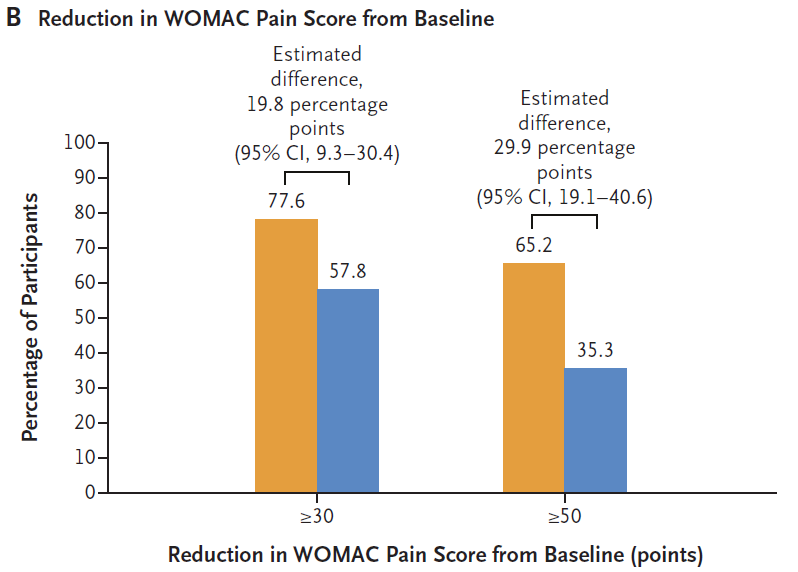
Comparison of the proportion of patients with pain scores at least 30% and 50% lower than baseline in WOMAC between the semaglutide group (orange) and placebo group (blue) at week 68 of treatment (Image source: Reference [1])
WOMAC's physical function score (17 items, each ranging from 0 to 10 points) decreased (improved) by 41.5 points and 26.7 points respectively compared to baseline (P<0.001)
In addition, at 68 weeks of treatment, the SF-36 physical function scores of the semaglutide group and the placebo group increased (improved) by 12 points and 6.5 points respectively compared to baseline (P<0.001); The waist circumference of patients in the semaglutide group decreased by 6.9% compared to the placebo group
The systolic and diastolic blood pressure in the semaglutide group decreased by 8 mmHg and 3 mmHg, respectively, which was higher than the 0 mmHg and 1 mmHg decrease in the placebo group.
In terms of safety, the incidence of serious adverse events in the semaglutide group and placebo group was 10% and 8.1%, respectively, with no significant difference. The common serious adverse event was gastrointestinal disease, and similar to previous related studies, no new safety events were found.
In summary, the results of this study indicate that semaglutide is more effective than placebo in reducing pain and weight in knee osteoarthritis, and is associated with improved physical function in patients.
CATEGORIES
News
- Welcome to the world of Tirzepatide, a w2025-02-28
- Is compound semaglutide safe?2025-02-25
- An average weight loss of over 20%! Tirz2025-02-24
- World Health Organization: GLP-1 such as2025-02-20
- Fortune: 'Weight loss miracle drug' sema2025-02-18
CONTACT US
Contact: NewPeptides
Phone: +852 6902 7583
E-mail: Linda@goodpeptides.com
Add: Science and Technology Industrial Park, Yuelu District, Changsha City, Hunan Province
